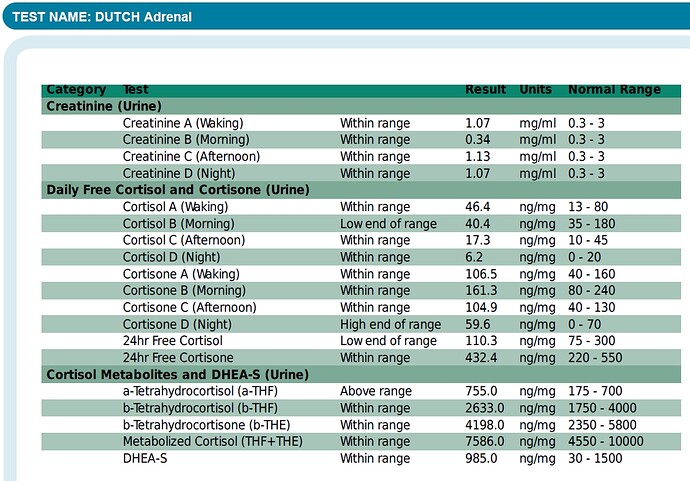
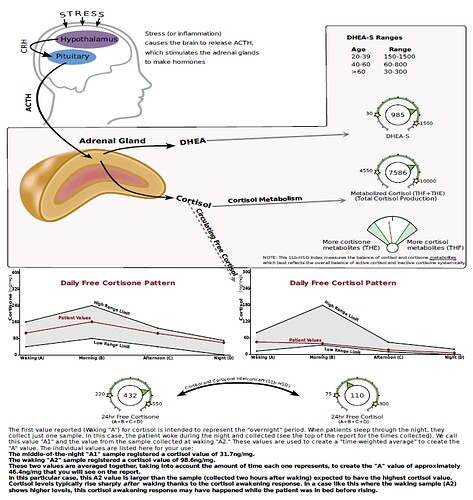
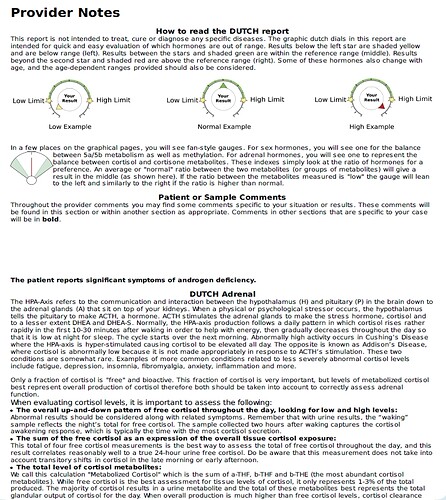
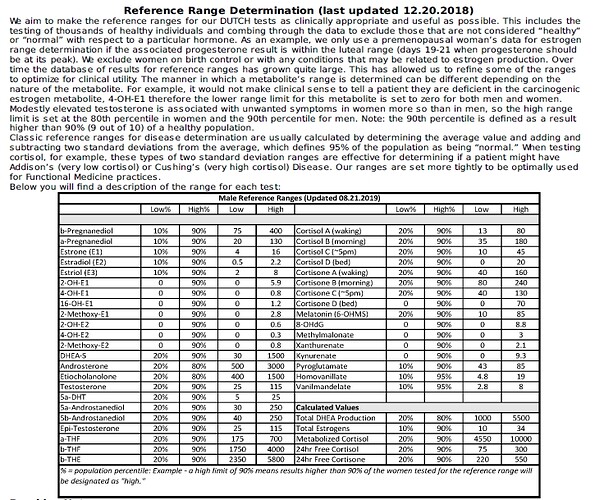
“Finasteride was originally thought to act as a competitive inhibitor with nanomolar affinity for 5α-reductase type 2 (12). More recently, it was found that finasteride acts as a mechanism-based inactivator of this enzyme”
“Subsequent to inhibitor binding, there is hydride transfer from the NADPH cofactor to the Δ1-2-ene double bond of finasteride”
“The intermediate enolate tautomerizes at the enzyme active site to form a bisubstrate analogue in which dihydrofinasteride is covalently bound to NADP+ The bisubstrate analogue has subnanomolar affinity for 5α-reductase type 2”
“Both AKR1D1 and 5α-reductase type 1 play important roles in the hepatic clearance of steroid hormones, suggesting that high dose finasteride may have an adverse effect on hepatic steroid metabolism”
“Inhibition of AKR1D1 by high dose finasteride would also deprive PXR of its natural 5β-pregnane ligands, resulting in diminished CYP3A4 induction”
“By contrast, in addition to being involved in bile acid biosynthesis, 5β-reductase is responsible for generating 5β-pregnanes, which are natural ligands for the pregnane-X receptor (PXR) in the liver”
“PXR is involved in the induction of CYP3A4, which is responsible for the metabolism of a large proportion of drugs (5, 6). Thus both 5α-reductase and 5β-reductase are involved in the formation of potent ligands for nuclear receptors”
“This could result in significant drug-drug interactions. Importantly, finasteride itself is metabolized by CYP3A4, suggesting that high dose finasteride might prevent its own metabolism (27)”
What if (AND THIS IS JUST A THEORY NOT BEING PRESENTED AS FACT) finasteride prevents it’s own metabolizing at least to a degree and if we had issues with PXR and or CYP3A4 that prevented it’s metabolizing and it’s still binding our NADPH. This is just a thought. And if it’s wrong that does not mean it’s smart to move on and not continue to take a closer look at NADPH
again these are just logical ideas and thoughts that may have been overlooked or not looked at close enough