Yes I have access to all publications. I think it’s always interesting that finasteride is interacting directly with these systems, but of course it doesn’t explain these elements of “PFS”. Rather, it shows a physiological interference in areas pertinent to PFS symptoms. It’s important to bear in mind the pattern of PFS on aggregate, and that many of our most severe cases completed suicides took very little of the drug and dramatically and progressively worsened following a crash hours, days, weeks later. And most importantly that millions are on this drug.
I have been interested in the PTSD overlap for a short while. I will maybe make a topic in a month or so. I spent some time looking into it a while ago after seeing a study (maybe @orthogs) posted. I’ve written a fair bit up in the literature review I’ve been doing and have found a lot I think people will be interested in reading. I don’t think it’s unreasonable to suggest as @frustrated has there may be some interesting symptomatic overlap and even potentially mechanistic. Certainly some amazing clinical stuff. Imo, this again highlights the mechanistic importance to medicine of this post-endocrine disruption syndrome.
In regard to this conclusion:
I don’t agree this experiment could support that conclusion if the androgen DHT is to be considered a gonadal steroid. To conclude this could be the case, it would have been important to conduct investigations into mRNA responses with administration of something like hydroxyflutamide. 5alpha reductase is a microsomal tissue-bound enzyme that allows for local synthesis site-specifically. It is also still very important in the female brain.
In terms of CRH, detailed mechanistic investigations currently in press (Heck and Handa, 2020) have demonstrated CHR mRNA is under the regulation of DHT, and evidence would suggest this is not due to gabergic neurosteroids.
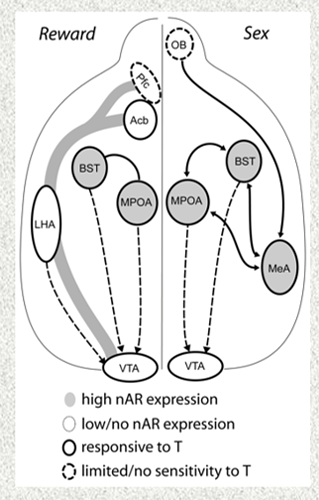
key: GDX = gonadectomy, projections = neurons with axons projecting to other sites
As this mechanism was highly likely to be AR mediated, Heck and Handa investigated and described robust AR labeling within and outside of CRH neurons in the BNSTav, "a brain region well positioned to mediate the androgen regulation of the HPA axis. Anterior subdivisions of the BNST have neurons with AR expressing projections to the CRH containing parvocellular regions of the PVN (78). Additionally, the BNSTav includes substantial populations of PVN projecting CRH neurons that are functionally distinct from GABAergic neurons in the region and have been suggested to participate in the activation of the HPA axis (79–81). DHT may enhance the activity of these BNST CRH neurons to increase Crh gene expression in the PVN in the absence of GCs.
Our findings show that GDX selectively decreases PVN Crfr1 expression when paired with ADX and support a role for CRH signaling in such androgen regulation of PVN Crh. Despite evidence for BNST to PVN CRH signaling, we cannot exclude the possibility that DHT inhibits the activity of GABAergic neurons in the BNSTav and other regions of the BNST that are not CRH expressing to increase PVN Crh expression following ADX. However, results of one study argued against such a mechanism of GABA disinhibition, as local DHT administration did not alter expression of GABA-synthesizing enzymes in the posterior BNST, a region thought to play an especially prominent role in the inhibition of the HPA axis (69)…Ultimately, the results of these studies have shown a sex difference in the regulation of PVN Crh that is revealed in the absence of GC-negative feedback and may depend on DHT actions outside of PVN CRH neurons in males. Our findings highlight the need for further systemic evaluation of central gonadal hormone effects on the HPA axis. Such studies will continue to advance our understanding of sex biases in the prevalence of stress-related pathologies."
Of interest for those aware of the curvilinear nature of androgen signaling: this study noted “the potentially dose-dependent, excitatory effect of DHTP observed in the current studies is not unprecedented, the possibility that DHT has opposing effects, if administered at high and low doses, is certainly intriguing”, noting dose dependent AR expression as a potential explanation for this paradox.
Seperately, supraphysiological androgen has been reported to predispose animals to depressive behaviour, causing a downregulation of AR in line with CRH mRNA. In the same study, orchiectomy caused a significant and correlated increase in AR and CRH (Ludwig et al 2019). Referencing animal and human studies of depression with supraphysiological androgens, they concluded “depression-like symptoms [may be] found on both ends of the spectrum…Altogether, it appears that long-term treatment with supraphysiological doses of testosterone induces depression-like symptoms in rats that were additionally exposed to uncontrollable stressors.”
Together, CRH is clearly under regulation of androgen signaling, and current evidence indicates this is AR mediated.