Hey guys, i lack time to properly introduce myself so i’ll save that for later.
So to keep it short;
Used 5-ar inhibitors (dutasteride, finasteride) in rotation for approx 2 years. Quit using the stuff 6 years ago after experiencing brain fog, cognitive impairment and gynecomastia but the symptoms never subsided and only got worse. I’m now diagnosed with chronic fatigue immune deficiency.
I also had gynecomastia surgery 2 years ago but the gyno came back! To get more insight into the cause of this whole ordeal i’ve been doing some extensive testing and much more to follow.
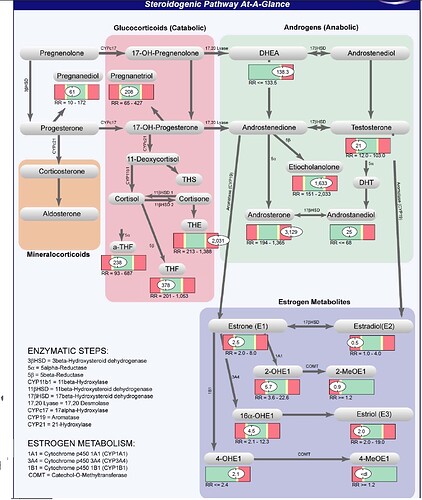
One of the tests i did is called Complete male hormones uriniray markers by genova diagnostics.
The strange thing is my estrogens are actually low and certain androgens very high! I was actually hoping my estrogens would be high explaining my gynecomastia but now i’m in the dark again. I know there are many more hormones to test but hopefully you guys can give me some advice on the outcome of the genova diagnostics test. Please see the screenshot below.
Other symptoms:
- Alopecia androgenetica (still)
- Memory loss
- Muscle waste / inability to gain weight
- Multiple gut issues, colitis, proctitis, ileitis, gastritis, GERD
- Hirsutism
- High libido
- Decreased semen volume
Commentary from genova diagnostics on my test results:
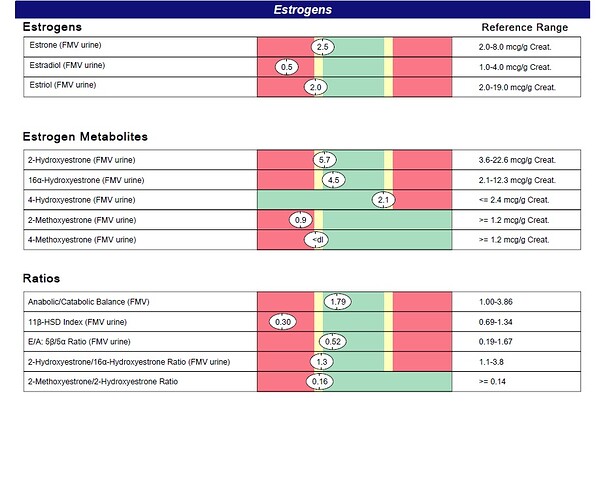
Estradiol (E2) is the most potent estrogen next to estrone and estriol, and is primarily formed in males from
testosterone via aromatization. Although E2 levels are significantly lower in men compared to premenopausal
women, men still require estrogen for cognitive function, as well as maintenance of bone density and various aspects
of cardiovascular function such as blood vessel health. Accordingly, low E2 levels are consistent with increased risk
of osteoporosis, endothelial dysfunction, and cognitive impairment. Low urinary E2 might result from reduced
androgens, broad-spectrum antibiotic treatment, or decreased conversion (aromatization) from androgen precursors,
including the use of aromatase inhibitors.
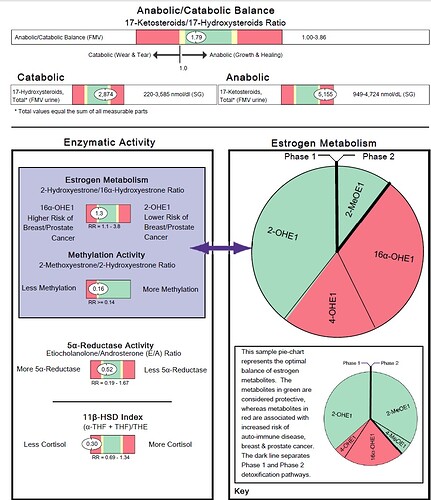
Conversion of 2-hydroxyestrone (2-OHE1) to 2-methoxyestrone (2-MeOE1) confers extra protection against prostate
cancer. 2-MeOE1 also possesses potent antioxidant properties. Low levels of 2-MeOE1 suggest impaired
methylation of 2-OHE1 or may simply accompany low levels of 2-OHE1. Methylation may be improved via methyl
donors such trimethylglycine, as well as cofactors such as folate, B6 and B12, and magnesium.
Low levels of 4-methoxyestrone (4-MeOE1) suggest impaired COMT-mediated methylation of 4-hydroxyestrone (4-
OHE1). Because 4-OHE1 is procarcinogenic and free radical inducing, 4-MeOE1 production is considered protective.
Methylation may be improved via methyl donors such trimethylglycine as well as cofactors such as folate, B6, B12,
and magnesium.
Like the 2-MeOE1:2-OHE1 ratio, the ratio of 4-methoxyestrone to 4-hydroxyestrone (4-MeOE1:4-OHE1) provides a
useful gauge of methylation capacity. A low ratio suggests impaired COMT-mediated methylation of 4-
OHE1. Because 4-OHE1 can convert to compounds that promote prostate cancer, a low 4-MeOE1:4-OHE1 ratio is
considered sub-optimal. Methylation may be improved via methyl donors such trimethylglycine, as well as cofactors
such as folate, B6, B12, and magnesium.
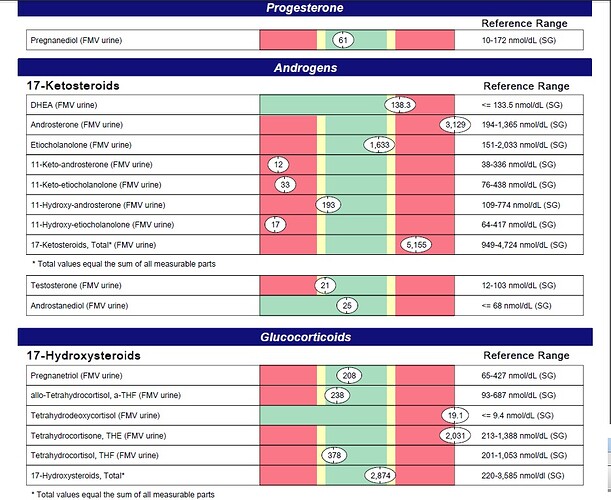
High levels of urinary DHEA are commonly due to excess supplementation, especially if urinary levels of total 17-
ketosteroids, androsterone and etiocholanolone are also elevated. High levels may also be due to a deficiency or
block of 3β-hydroxysteroid dehydrogenase enzymes.
Tetrahydrodeoxycortisol (THS) is a minor derivative of 11-deoxycortisol, which, in turn, is formed from progesterone.
11-deoxycortisol is also a precursor of cortisol via 3β-hydroxysteroid dehydrogenase (3β-HSD). High levels of THS
might thus result from excess levels of 11-deoxycortisol and/or progesterone, such as might occur with progesterone
therapy. High THS levels might also be expected from excessive adrenal stimulation, an enzymatic block in 11β-HSD
(expect low cortisol), adrenocortical carcinoma, and/or pituitary dysfunction. Confirm with other lab values.
Tetrahydrocortisone (THE) is a direct metabolite of cortisone. High levels of urinary THE are usually due to exogenous
sources. Excess cortisol and/or increased conversion of cortisol to cortisone (such as occurs in hyperthyroidism) might
also result in high THE levels. Confirm with other lab values.
The 11β-HSD Index is a ratio of the cortisol metabolites tetrahydrocortisol (THF) plus allo-tetrahydrocortisol (a-THF)
divided by tetrahydrocortisone (THE). It is a reflection of 11β-hydroxysteroid dehydrogenase (11β-HSD) enzyme
activity. This enzyme catalyzes the inactivation of cortisol or the activation of cortisone within the peripheral tissues
such as the adipose, liver, bone and nerve. Intracellular cortisol/cortisone equilibrium has been shown to be as
important as the circulating concentration of cortisol represented by the Urinary Free Cortisol level and may be a key
player in the modulation of cellular metabolism associated with the Metabolic Syndrome. Ratios less than 1 indicate
that cortisol is being inactivated to cortisone by 11β-HSD-2 in the peripheral tissues. This pattern has been noted in
hyperthyroidism as well as increases in growth hormone.
Androsterone is an androgenic downstream metabolite of DHEA and androstenedione via 5α-reductase, and may also
be formed directly from androstanediol, a metabolite of DHT. Elevated levels of androsterone usually occur with DHEA
administration (confirm with elevated DHEA, etiocholanolone and total 17-ketosteroids). High levels have also been
noted in hyperthyroidism, in women with polycystic ovary syndrome (especially when obese), hirsutism, and/or acne,
and in men with benign prostatic hypertrophy.
Low levels of 11-keto-androsterone may be due to an androstenedione deficiency. Low DHEA and/or cortisone may
also contribute. Confirm with urinary DHEA and tetra-hydrocortisone levels.
Low urinary 11-keto-etiocholanolone levels are generally associated with androgen deficiency. This is a 5β-reduced
compound that is an end product of androgen catabolism.
Low levels of 11-hydroxy-etiocholanolone are usually due to androstenedione deficiency. However, low DHEA
(precursor of androstenedione) can also contribute to low levels. Cortisol is a minor contributor but a deficiency may
affect values. Confirm with urinary cortisol and DHEA levels.
The 5β / 5α Reductase (Etiocholanolone/Androsterone) Ratio is a sensitive indicator of the 5β-reductase vs. 5α-
reductase enzyme activities, as both androgen metabolites come from androstenedione via these two enzymatic
pathways. Etiocholanolone is produced via the 5β-reductase pathway, thus a ratio of less than 1 suggests a shift to
the 5α-reductase pathway. A lower ratio (indicating more 5α-reductase activity) has been observed in individuals with
polycystic ovary syndrome, obesity, male-pattern baldness, as well as with an intense athletic training and
consumption of a western diet. A ratio of 1 might suggest excessive tissue dihydrotestosterone (DHT), especially with
testosterone supplementation (expect higher urinary androstanediol level).
The Anabolic / Catabolic Balance is a ratio of the total 17-ketosteroids (anabolic) to the total hydroxysteroids
(catabolic). This ratio reflects the balance of growth and healing versus wear and tear in the body. A ratio greater than
1 demonstrates a net anabolic state for the patient, a beneficial finding.
High levels of urinary 17-ketosteroids occur with excess DHEA supplementation. However, elevated urinary 17-
ketosteroids may also result from high levels of testosterone (if urinary androsterone and etiocholanolone are also
high) or excessive supplementation of dihydrotestosterone, androstenedione, or growth hormone. A high protein and
high calorie diet may also elevate urinary 17-ketosteroids, as can various agents such as antibiotics. Rule out
pregnancy, tumors of the adrenals, ovary or testes, pituitary dysfunction, Cushing’s disease, hirsutism (if also low to
normal 17-hydroxysteroids), PCOS, and hypertension.