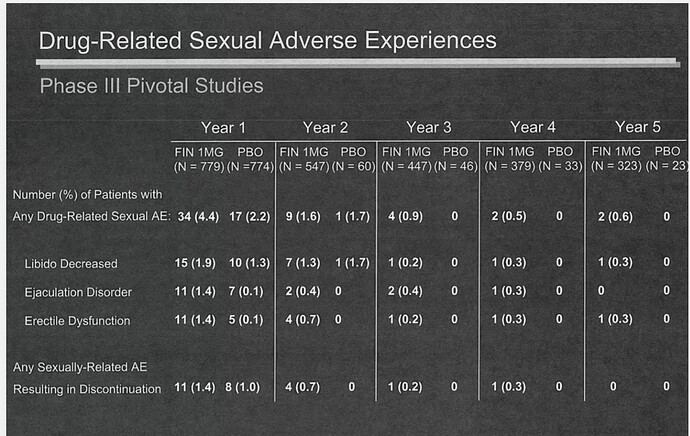
The number of people who taken proprcia (N) in the table is decreasing every year. Are they dropping out because of side effects?Why is no one questioning Merck’s stduy?
This was a point Reuters made in their report. The way Merck has reported their data is not “fair or standard practice”, and it’s impossible to tell exactly how many men experienced side effects during their trials.
From the memos and emails they discovered, however, there was definitely 29 that were not reported. I doubt it was hundreds as there are many reasons people drop out of studies, particularly five-year studies like this one.
Our group is creating some content around this which we’ll be releasing when we launch our new charity, along with the new podcast series, which will break down the trials and what Reuters found in more detail. I’ve read hundreds of pages of the docs in excruciating detail and it’s quite scandalous and surprising it wasn’t reported on more widely.
Do you have links to their memos and emails?Does it say 29 people weren’t reported? I’d like to see it
I believe you can find them on the Foundation’s website: https://www.pfsfoundation.org/propecia-litigation-library/ in the emails from Patrick Ruane to Keith Kaufman. You have to piece it together as it’s not explicitly stated, but they talk about sexual adverse events in an email.
This is one of the reasons we talk about the importance of reporting to regulators. If adverse events, particularly persistent ones, go underreported, regulators will never be forced to act and these clinical trials will be forever cited as proof of finasteride’s safety.