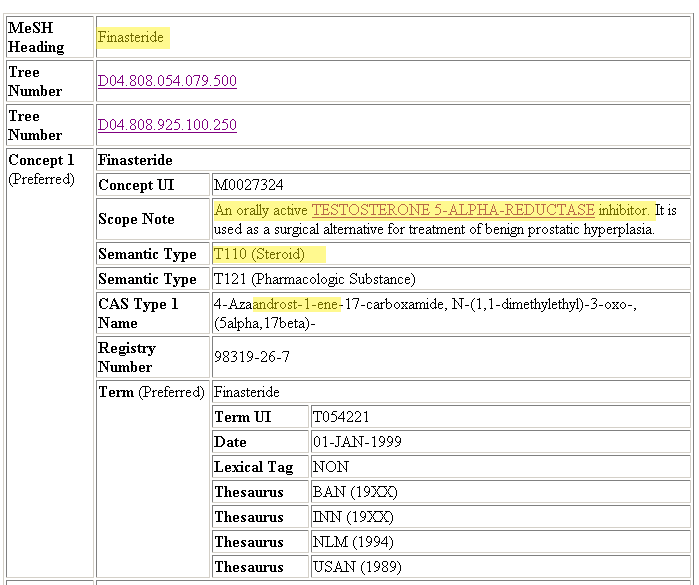
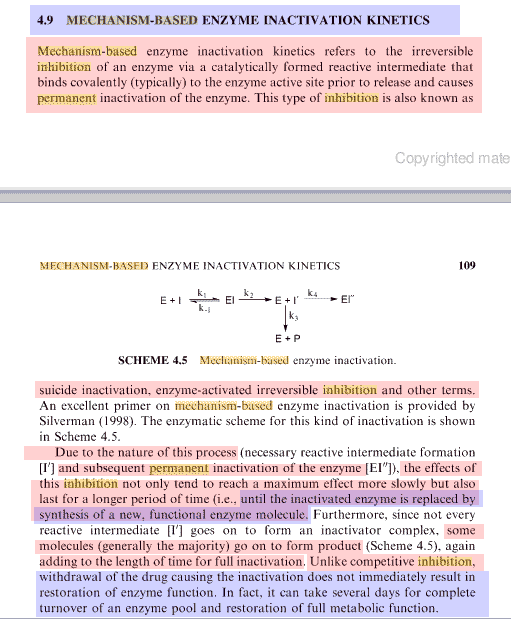
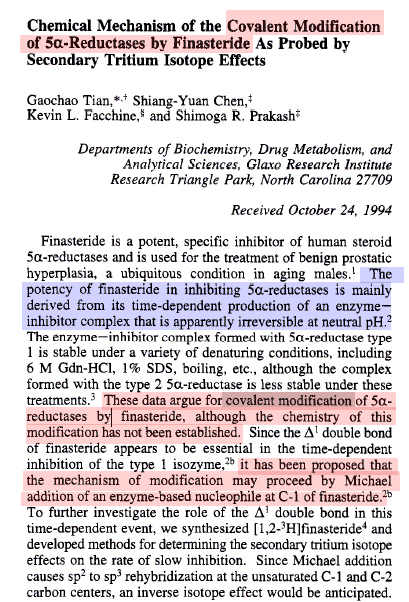
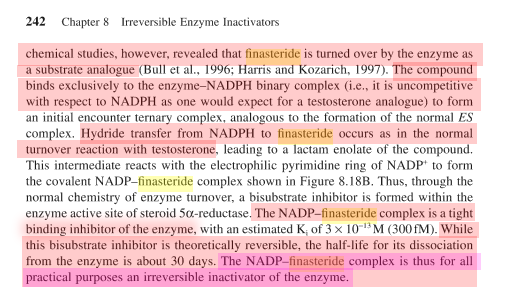
Note: Mechanism-based inhibition = suicide inhibitor
simcyp.com/ResearchDevelopme … echenzyme/
"Mechanism (or time) -based enzyme inhibition is associated with irreversible or quasi-irreversible loss of enzyme function, [Size=4]requiring synthesis of new enzyme before activity is restored[/size].
The consequences of mechanism-based inhibition are:
- auto-inhibition of the clearance of the inactivator itself
- prolonged inhibition of the clearance of other drugs that share the same enzyme.
There may also be serious immunotoxicological consequences if a reactive intermediate is covalently bound to the enzyme. Therefore, screening of new compounds for mechanism-based enzyme inhibition is now standard practice within the pharmaceutical industry"
lib.bioinfo.pl/pmid:9749833
Cloning, expression and characterization of rhesus macaque types 1 and 2 5alpha-reductase: evidence for mechanism-based inhibition by finasteride.
The rhesus macaque types 1 and 2 5alpha-reductase (5aR1 and 5aR2) were cloned and expressed in COS cells to facilitate comparison of rhesus and human 5aRs. The deduced protein sequences of the rhesus SaRs shared 94% and 96% identity with the human type 1 and 2 isozymes, respectively.
Despite a four amino acid insertion at the N-terminal region of rhesus 5aR1, the biochemical properties of rhesus and human homologs are very similar with respect to pH optimum, Km values for testosterone and progesterone, and inhibition by a variety of inhibitors.
As expected, the biochemical properties of the human and rhesus 5aR2 are also very similar. The mechanism of inhibition of the rhesus 5aR1 and 5aR2 by finasteride was investigated in more detail.
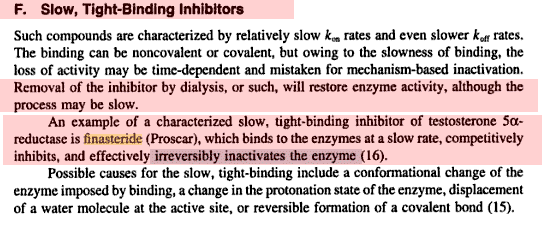
Finasteride displays time dependent inhibition of the rhesus 5aR1 and 5aR2 with second order rate constants of 4 x 10(3) M(-1) s(-1) and 5.2 x 10(5) M(-1)s(-1). Inhibition of rhesus 5aR2 with 3H-finasteride resulted in 3H bound to the enzyme which is not released by dialysis.
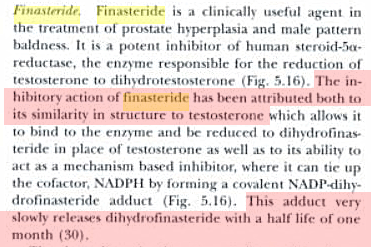
Heat denaturation of the [rhesus SaR2:inhibitor] complex releases dihydrofinasteride, a breakdown product presumably related to the NADP±adduct previously identified with the human SaRs (Bull et al., Mechanism-based inhibition of human steroid 5alpha-reductase by finasteride: Enzyme catalyzed formation of NADP-dihydrofinasteride, a potent bisubstrate analog inhibitor. J. Amer. Chem. Soc., 1996, 118, 2359-2365).
Taken together, these results provide good evidence that the rhesus macaque is a suitable model to evaluate the pharmacological properties of finasteride and other 5aR inhibitors.
Attached image from:
books.google.ca/books?id=7JrFamz … #PPA109,M1