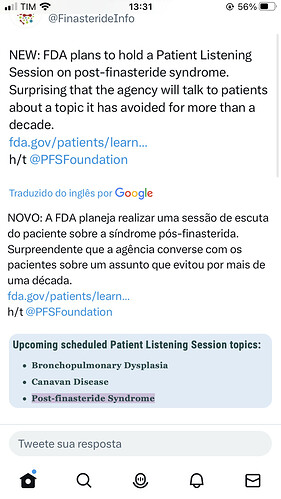
Enter twitter and RT this guys
FDA Link: https://www.fda.gov/patients/learn-about-fda-patient-engagement/fda-patient-listening-sessions
The Tweet: https://twitter.com/FinasterideInfo/status/1639640463232696320?s=20
Really pleased that a volunteer helping out with our efforts, @wonk, was able to organise this. We’ve already put together the presentation including testimonials from 8 patients and a thorough overview of the disease, which we’ll be presenting in two weeks. Unfortunately the schedule is full up though as we only get two hours.
Also worth noting the purpose of this is not asking for regulatory action. Although this is the FDA, the group running these sessions is not a regulatory group and is just seeking to learn more about rare disease groups and the challenges they experience. Either way, it’s great to be on their radar.
We’ll report back with updates after the session.
I just saw this news on Twitter. Thank you for the efforts of American patients, and I look forward to more information about it.
Will it be recorded or streamed anywhere? Interested in viewing this.
despite the purpose of sit down, we should at least make an ask that would speed up our timeline to recovery if granted.
they will likely say no but we should ask regardless just to see whats possible and what their response is
This. Yes.
When is this FDA meeting happening?
It’s in a couple of weeks.
Did this happen??
The other two items listed in the OP for upcoming sessions are both on their summaries page as having taken place. I don’t see PFS in either the upcoming or came-and-went lists.
According to the fda patient Twitter account apparently the listening session happened yesterday 
??? Did we have no participation??
The FDA listening session took place yesterday and it was overall a positive experience. The attendance was apparently quite high and all who attended were respectful.
Thank you to all who spoke out, including Denise Turner.
There isn’t really any clear objective for the listening session beyond having an audience with the FDA, so it’s unclear what the outcome will be now.
We’ll keep the community updated.
I would like to join @Sugarhouse in thanking everyone who participated in the session. Overall I was quite pleased with the presentation from our side as well as the audience/participants from the FDA.
A thorough summary of the meeting is now available here: https://www.fda.gov/patients/learn-about-fda-patient-engagement/patient-listening-session-summaries