hello here is a mail i sent to MHRA :
Dear Peter and Doctor Henderson,
Thank you for your reply .
I would allow myself a few comments and clarifications on teragonicity in part 1), and on genetic polymorphisms in part 2), and I would still have a few other important questions in bold.
- teratogenic risk:
Concerning my question 3 on teratogenicity, and your answer saying that the 1 month period is accepted worldwide, I would say that the absence of proof is not proof of the absence of a real, underestimated and poorly studied.
Knowing that sodium valproate is the second teratogen, the 1st being isotretinoin on the list of teratogens.
Knowing that a French study was carried out and published in 2021 concerning depakine “Transgenerational adverse effects of valproate? A patient report from 90 affected families” which you will find here: https://onlinelibrary.wiley.com/doi/abs/10.1002/bdr2.1967
« summary :
Context
Use of valproate during pregnancy increases the risk of malformations and neurodevelopmental disorders. Experimental data in mice have shown that valproate is a direct inhibitor of histone deacetylase, inducing histone hyperacetylation, histone methylation and DNA demethylation, which causes birth defects with epigenetic inheritance . We investigated the potential transgenerational adverse effects of valproate.
Methods used
We interviewed 108 people (from 90 families) suffering from complications due to exposure to valproate in utero and who were themselves parents (85 women and 23 men) about the occurrence of malformations and neurodevelopmental disorders in their children. All were members of Aid to Parents of Children with Anticonvulsant Syndrome (APESAC), a charity established in 2011 to provide personalized help and support to families suffering from complications due to exposure to valproate during the pregnancy.
Results
Among their 187 children, they reported 43 (23%) children with malformation(s) (26 hand or foot malformations; 15 facial dysmorphisms; 10 renal/urological malformations; 6 spina bifida; 4 cardiac malformations; 2 craniosynostoses; 2 cleft lip and palate) and 82 (44%) children with neurodevelopmental disorders (63 problematic behaviors and autism; 41 psychomotor disorders; 16 language problems; 16 attention deficits; 5 mental retardations). Only 88 (47%) children presented neither malformation nor developmental disorder.
Conclusion
These data reinforce the need to fund pharmacoepidemiological investigations into epigenetic inheritance caused by drugs causing malformations or neurodevelopmental disorders. People exposed in utero to valproate should be informed of the risk, so that they can consider fertility options, prenatal diagnosis and adequate early monitoring.
Conflict of interest :
Marine Martin created the association “Aid for Parents of Children Suffering from Anticonvulsant Syndrome” (APESAC https://www.apesac.org/ ) in 2011 and initiated legal proceedings. »
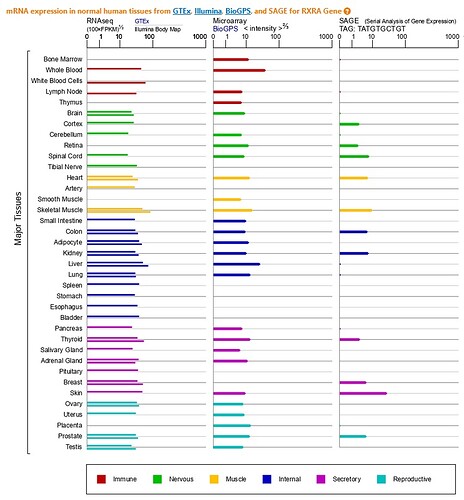
if you reread from page 17 the document addressed to the ANSM, translated as an attachment, you see on the graphs from the genetic databases that the RXRalpha receptors (RXRA) are overexpressed, among others, in cells germinal and sexual. Letter that I address to you also again.
If we query this genetic database: Genecards - RXRA (Retinoid X Receptor Alpha)
I would also like to point out that
Isotretinoin has three levels of presence in the body:
a) short term: half-life of 46 to 48 hours (50% reduction in plasma level)
b) medium term: about a month at the intracellular detection limit (except RNA) - then, the “good” effects start to dissipate and acne and sebum can potentially return
c) long-term: up to a year to eliminate RNA and stop the regulation of gene transcription
References: https://www.ncbi.nlm.nih.gov/pubmed/9427083
Due to c) and its huge impact on HOX gene transcription), you should not become pregnant for a full year after stopping treatment. At least 6 months, but the safest choice is a year.
Indeed, if we do not consider the genetic aspect but only the elimination of the product in the blood, 1 month of delay may seem logical, but if we broaden the reflection towards a more holistic analysis, by adding to the percentages essential factors such as genetic polymorphisms, and if we also widen the time window: the transgenerational aspect must be taken very seriously. Especially given what we know about the second teratogen just after the worst and the first of the teratogens: isotretinoin…
The importance of the genetic aspect, despite the lack of studies and consideration of this aspect, and by extension of the transgenerational effect, presents an extremely strong and intricate analogy with the problem of long-term damage in one’s life.
Question 1 :Do you still think that only 1 month of waiting before a pregnancy is justified, and would not deserve to be reconsidered in view of these elements?
These 2 problems arise from genetic polymorphism, which alone explains the heterogeneity of responses to siotretinoin treatment, a genetic polymorphism that you also mention in your report.
- Genetic polymorphism and mutations:
Although since 2004 at least, several people have been warning of the imperative need for a declaration of mutagenesis (see pages 17 to 25 in the email for the ANSM, which also concerns you, where a reflection on a way to improve is detailed clinical practice).
According to Khabour et al, 2018, the association between the rs7799039 polymorphism of the leptin gene and changes in the lipid profile induced by isotretinoin treatment could modulate the
lipid parameters and liver enzymes.
According to M Garba 2020, The association between the single nucleotide of adiponectin in connection with the polymorphisms and side effects of isotretinoin in acne patients studies the ADIPOQ gene which carries rs1501299 and rs2241766, the sample size and variations due to diet require the study to be reproduced on other populations, and a larger cohort.
In your report, page 26:
“3.1.2. Genetic polymorphisms
Genetic polymorphisms or mutations of components of the ATRA Fox03 TRAIL death signaling cascade may explain the observed individual susceptibilities enhancing isotretinoin-mediated apoptosis.
Genetic polymorphisms in the RARA gene have been associated with an increased risk of adverse effects of isotretinoin (Alzoubi et al., 2013). Analysis of three-locus haplotypes (rs2715554 C/T rs4890109 G/T rs9303285 T/C) showed that the frequencies of the CTG and TTG haplotypes are significantly associated with the occurrence of arthralgia, myalgia, nosebleeds and headaches in patients treated with isotretinoin. These side effects are all listed in the product information as possible side effects associated with isotretinoin.
Additionally, the TCG haplotype was associated with nosebleeds and headaches, while the TTT haplotype was associated with arthralgias and myalgias.
Aspartate aminotransferase (AST) levels were increased in patients with the TC genotype of the rs2715554 polymorphism, while the T allele of rs9303285 was found to be protective against the development of depression in patients treated with isotretinoin (Alzoubi, et al., 2013).
As such, genetic variations in critical regulators of isotretinoin-induced apoptotic signaling may explain subgroups at increased risk of side effects or treatment resistance while receiving systemic isotretinoin. Melnik hypothesized that screening for gene polymorphisms that increase susceptibility to isotretinoin-induced apoptosis and isotretinoin-associated depression could be useful in identifying individuals with increased isotretinoin-mediated apoptotic signaling. isotretinoin in the future (Melnik, 2017).
Genetic polymorphisms or mutations of components of the ATRA Fox03 TRAIL death signaling cascade may explain the observed individual susceptibilities enhancing isotretinoin-mediated apoptosis.
In summary, one of the main mechanisms underlying the mode of action and adverse effects of isotretinoin is apoptosis. The extent of isotretinoin-induced apoptotic signaling may depend on genetic variations, such as RARA polymorphisms. The data provide additional information on the mode of action of isotretinoin, its risk profile and may at least partly explain the increased sensitivity to adverse reactions
individuals linked to apoptosis in the subgroup of people with genetic variations in isotretinoin-induced apoptotic signaling pathways. »
“Mutations of this enzyme lead to biotin deficiency (Bremner, et al., 2005), (Csoka & Szyf, 2009) . Administration of isotretinoin to humans is associated with a decrease in biotinidase (Ding, Kam, Dieckow, & Sullivan, 2013), and the presumed resulting decrease in biotin may contribute to depression. » (page 31)
“3.2.1.1.3. Genetic polymorphisms
The effect of genetic polymorphisms (variations) of the RARA gene has been studied for the relationship with depression.
In a study of 300 patients treated with oral isotretinoin, it was shown that the T allele of rs9303285 protected against the development of depression and that the rs2715554 and rs54890109 polymorphisms were not associated with the development of depressive symptoms during treatment. use of isotretinoin (Alzoubi, et al., 2013). However, this study requires further validation to assess possible associations and explore whether other, as yet unidentified, factors have a role in the clinical outcome of oral isotretinoin. There are currently no commercially available tests for screening for these polymorphisms. »
Question 2 :You therefore specify in the report, however, that no test exists to detect these risk genotypes in clinical practice. Can you give me scientific sources or details on this assertion?
Question 3 : Do you confirm or deny that an RT-PCR test can detect these risk genotypes, by focusing on these polymorphisms?
If so, we all know that private companies in the USA do genomic sequencing for sums (paltry, compared to the cost of lifelong disability for a state) ranging from $70 to $300.
One final note and question: About 25 percent of your report discusses tangible and genetic mechanisms, which for me is one of the first relevant and honest reports
that I have read at this level, and from my point of view, and 75 percent of your report discusses the link or not between isotretinoin and depression (and sexual effects).
The proportions of symptoms discussed in these 75 percent of your report are not at all proportional to the related pharmacovigilance signal (1/3)…
The gastrointestinal effects are as numerous (1/3) as the “depressions”, and are barely mentioned (yet medications to treat acne or iatrogenics are well digested in the gastrointestinal system), not to mention all autoimmune diseases, invisible, and yet arthralgia, myalgia, nosebleeds and headaches are significantly associated with the CTG and TTG haplotypes.
These symptoms are also closely linked to fibromyalgia, myalgic encephalitis, rheumatoid arthritis, Gilbert syndrome, Chron, inflammatory bowel disease, etc…
Question 4: with all the respect I have for people of good faith and good will, would the depression and psychiatric disorders observed not rather be a second-order consequence (as is often the case with serious illnesses) of physical and organic diseases, iatrogenic and bothersome, chronic, painful, invisible and little recognized, even constantly despised by the medical profession, associated with the psychosocial result of the resulting disability (and the stigmatization that goes with it), in addition very often to the incomprehension and the feeling of helplessness, caused by these genetic polymorphisms (which by definition are not representative of all patients, and therefore which cause additional isolation and misunderstandings), which can either resemble or lead to similar -depressions and suicides, but intrinsically, are due to genetic mutations and anarchic and more than useless apoptosis?
The eHealthMe phase 4 study goes in this direction.
Let’s not waste time, (money), and energy, and intellectual probity, for 39 years discussing this link between depression and isotretinoin, which due to the questionable nature, diffuse and vague nosologically, and as a consequence of his anamnesis: does depression always lead to a dead end?
In my opinion, your report highlights the few tangible markers available to diagnose (non-invasively) psychiatric disorders in comparison with other side effects, which have tangible biological, and even genetic, markers.
We must face the facts… because serotonin is never tested before prescribing antidepressants, because it seems that it only passes the blood-brain barrier in one direction, like the Chernobyl cloud which stops at the borders…but that’s not the point.
Does this not amount to carefully studying for 39 years a tree or a forest edge masked by a thick screen of smoke (link between depression and isotretinoin) which hides an immense forest in the process of burning (polymorphisms and genetic mutations from which result iatrogenic ORGANIC diseases)?
Forest of inflamed cells that you had the merit of considering in depth, and I thank you for that.
thanking you
Kind Regards