2006 - A new look at the 5alpha-reductase inhibitor finasteride.
ncbi.nlm.nih.gov/entrez/quer … s=16834758
FULL PDF: blackwell-synergy.com/doi/pd … 06.00053.x
Finasteride is the first 5alpha-reductase inhibitor that received clinical approval for the treatment of human benign prostatic hyperplasia (BPH) and androgenetic alopecia (male pattern hair loss).
These clinical applications are based on the ability of finasteride to inhibit the Type II isoform of the 5alpha-reductase enzyme, which is the predominant form in human prostate and hair follicles, and the concomitant reduction of testosterone to dihydrotestosterone (DHT).
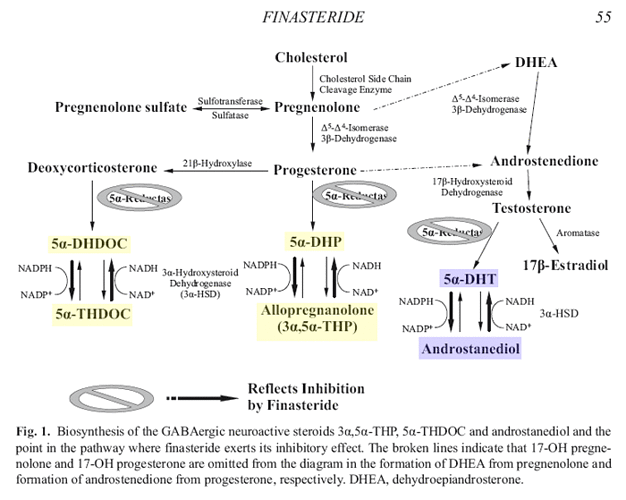
In addition to catalyzing the rate-limiting step in the reduction of testosterone, both isoforms of the 5alpha-reductase enzyme are responsible for the reduction of progesterone and deoxycorticosterone to dihydroprogesterone (DHP) and dihydrodeoxycorticosterone (DHDOC), respectively.
Recent preclinical data indicate that the subsequent 3alpha-reduction of DHT, DHP and DHDOC produces steroid metabolites with rapid non-genomic effects on brain function and behavior, primarily via an enhancement of gamma-aminobutyric acid (GABA)ergic inhibitory neurotransmission.
Consistent with their ability to enhance the action of GABA at GABA(A) receptors, these steroid derivatives (termed neuroactive steroids) possess anticonvulsant, antidepressant and anxiolytic effects in addition to altering aspects of sexual- and alcohol-related behaviors.
Thus, finasteride, which inhibits both isoforms of 5alpha-reductase in rodents, has been used as a tool to manipulate neuroactive steroid levels and determine the impact on behavior.
Results of some preclinical studies and clinical observations with finasteride are described in this review article.
The data suggest that endogenous neuroactive steroid levels may be inversely related to symptoms of premenstrual and postpartum dysphoric disorder, catamenial epilepsy, depression, and alcohol withdrawal.