I have appointment with my doc next week and will ask him to prescribe me mifepristone. In the following summary of scientific literature I explain why.
HPA Hyperactivity
Allopregnanolone (Allo) is important for the pregnant mother. Indeed, during pregnancy, an increase of Allo levels occurs in the maternal peripheral circulation, as well as in the maternal brain. In rats, the increased levels of this neuroactive steroid interfere with the hypothalamic-pituitary- adrenal (HPA) axis reducing, in particular during late pregnancy, the response to stress exposure of the mother.[1]A study showed that there is an apparent correlation between endogenous levels of brain allo and basal and stress-stimulated HPA axis activity.[2]
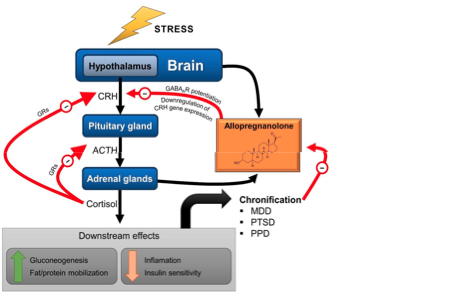
This could mean high Allo surpresses the HPA axis and low Allo, as reported in PFS, leads to HPA Hyperactivity. Definetely, HPA Hyperactivity is reported after the use of isotretinoin .[3],[4] Normally there is the negative feedback loop thorugh glucocorticoids at the Glucocorticoidreceptor (GR). However after taking Iso, the Glucocorticoid Dexamethsone does not surpress the plasma concentration of the glucocorticoid Corticosterone.[5] This means that the negative feedback loop is impaired. Histological examination and western examples affirmed this showing a significant decrease of GR-IR cells and GR density. The other pathway in which Isotretinoine increases HPA activity is an increase in the CRH-expression. This is through RARalpha activation, which could upregulate CRH mRNA expression via direct action on its promoter sequence.[6]
In general HPA Hyperactivity leads to more Glucocorticoids being produced.
The Glucocorticoids intervene with the secretion of GnRH. Glucocorticoids modulate the HPG axis by directly inhibiting the release of GnRH from the hypothalamus and the synthesis
and release of gonadotropins from the pituitary.[7] GnRH has the task to stimulate FSH and LH production. If it´s inhibited less FSH and LH will be produced.
The HPA Hyperactivity with all histological changes could be normalized by Mifepristone.[3]
Safety of Mifepristone
The mifepristone studies are relatively unique in that subjects received the medication for 7 days. Summary of seven clinical trials, five of them double blind (N ranging from 5 to 433), suggests that mifepristone has efficacy in reducing psychotic symptoms.Importantly, mifepristone’s effectiveness appears to be optimized when attaining a plasma level of ~1600ng/mL, which equates to roughly 1200 mg/day orally.
Patients with high mifepristone plasma levels also showed a reduction of depressive symptoms relative to placebo. Although mifepristone is associated with some gastrointestinal side effects and headache, very few patients discontinued due to side effects.
A metaanalysis said the following: Adverse Events (AE) from all five studies ( n = 1460) were evaluated by dose group and by all doses combined.
Overall, the safety of mifepristone versus placebo was comparable, with a similar number of events noted in each group. The NNH (Number needed to harm) was calculated using the outcome of study dropouts due to AEs. For all studies combined, the NNH for mifepristone was incalculable because dropouts due to side effects were higher for placebo (1.6%) than for mifepristone (1.4%). In two studies, dropouts because of AEs were higher for mifepristone than placebo (1.0% vs. 0.9%, NNH = 1000 in study 1; 2.4% vs. 1.6%, NNH = 126 in study 3). Mifepristone appeared safe and well tolerated across all three dosage groups. The rates of treatment emergent adverse events were similar across placebo (62%), mifepristone total (67%), mifepristone HPL (69%), and mifepristone LPL (65%) groups. There was no statistically significant difference in rates of adverse events between mifepristone HPL and LPL groups.[8]
References
-
Central Opioid Inhibition of Neuroendocrine Stress Responses in Pregnancy in the Rat Is Induced by the Neurosteroid Allopregnanolone, https://www.research.ed.ac.uk/en/publications/central-opioid-inhibition-of-neuroendocrine-stress-responses-in-p
-
Allopregnanolone modulation of HPA axis function in the adult rat, https://pubmed.ncbi.nlm.nih.gov/24658404/
-
All- trans retinoic acid-induced hypothalamus–pituitary–adrenal hyperactivity involves glucocorticoid receptor dysregulation, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4030330/
-
Chronic all-trans retinoic acid administration induced hyperactivity of HPA axis and behavioral changes in young rats, https://pubmed.ncbi.nlm.nih.gov/20659790/
-
All- trans retinoic acid-induced hypothalamus–pituitary–adrenal hyperactivity involves glucocorticoid receptor dysregulation, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4030330/
-
Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5438761/
-
Retinoic acid and depressive disorders: Evidence and possible neurobiological mechanisms, https://www.sciencedirect.com/science/article/abs/pii/S0149763420300014
-
Combined Analysis of Mifepristone for Psychotic Depression: Plasma Levels Associated with Clinical Response, https://www.biologicalpsychiatryjournal.com/article/S0006-3223(18)30035-0/fulltext

